According to Padraic Cyril Mc Freen, “Linked Inclusion™ is the correct next step in social media. We are excited about the potential for the platform and the response from early adopters has been overwhelmingly positive. Our vision is to support the Linked Inclusion™ community by providing the resources, time and attention finely tuned to the needs of its membership. Everyone is welcome.”
MiVu® President and CIO, Padraic McFreen, Supports Nokia® President and CEO, Pekka Lundmark's, Stabilization Plan Forward
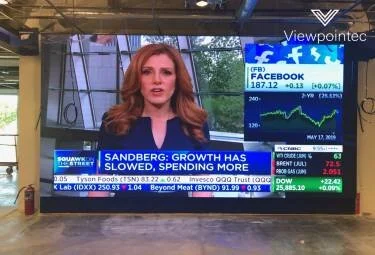
MiVu® and Viewpointec® Ink Strategic Agreement—Immersion Technology MiVu® AmFlex ImmersiVu™
MiVu® announces another strategically important partnership with Viewpointec®, the leader in LED Display Technology. Together, MiVu® and Viewpointec® introduce MiVu® AmFlex ImmersiVu™. The two have inked an agreement to jointly develop, market and deploy technology leveraging Viewpointec®’s patented Flex LED technology and MiVu®’s rapidly evolving Smarter™Network Hardware, Software and Services.
MiVu® and BlackVue® Join Forces to Significantly Improve Vehicle Safety
40 Days: MiVu® Cloned
MiVu became “Big Tech’s Best Kept Secret”, while SBC and all that followed, unwittingly adopted and used MiVu’s “Black tech startup origin identifier” to promote the MiVu Network, Phase One, as SBC, The Premier Communications and Entertainment Services Provider.
MiVu® and Viewpointec® Near Deal To Develop Smarter™ Network LED Technology
40 Days: Powered By MiVu® — Pt. II
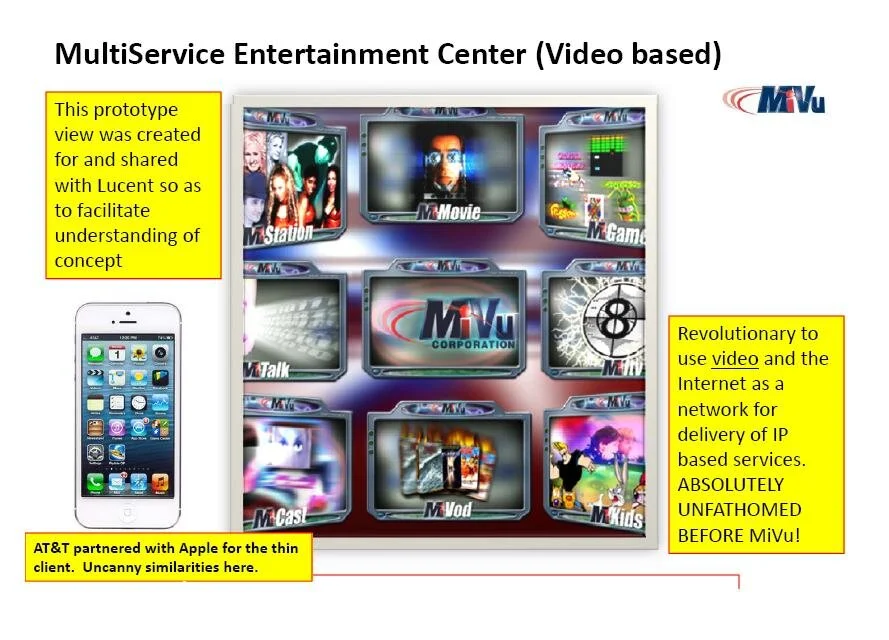
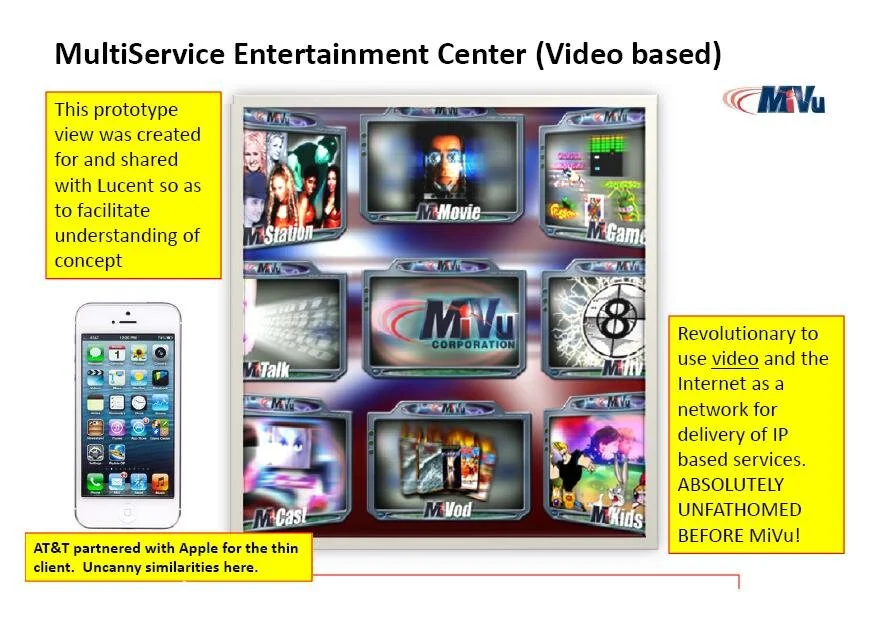
MiVu, the culmination of Padraic McFreen’s experiences, rather than becoming his biggest triumph, had alternatively become his biggest dilemma: “change the world just so, so as to just change the world; or change the world and also the world of those changed.” This author believes that’s Genius speak for, “between a rock and a hard place.”
40 Days: Powered By MiVu®
40 Days: MiVu® Revolutionized “The Last Mile”
40 Days: MiVu® Is the Alpha of Smart and Digital
Not clear when the Genius of Bell Labs became super envious of the Genius of MiVu, but as I mentioned, Bell Labs changed the name of Padraic McFreen’s work from MiVu, to MiLife, staking claim to the, then, MiVu Services and the MiVu Marketplace (remember the “neutral exchange”, well think AppStore) tag line, “all things important in my life right here
40 Days: MiVu® Broke Every Rule In Every Book
Keep in mind, Padraic McFreen’s startup launched in September of 2000. Here he is briefing Bell Labs in early October 2000 and Bell Labs is taking it serious — so serious it issues a request for interest and in that moment set in motion the creation of the Internet Industry valued today at approximately $11 Trillion USD.
40 Days: MiVu® Was Just What We Needed
This author can say with confidence, that Genius is a “spark.” It’s “light” in the pitch of darkness. Genius is the Ether that gives “Eureka” moments a foundation upon which to form and flourish.
You just know you’re in the presence of someone that has thought a lot about a lot of big problems facing humanity and has worked out the possible permutations.
40 Days: MiVu® Made The Internet Not Only Cool Again, But Gave It Purpose
40 Days: MiVu® Changed Everything Forever
40 Days: Powered By MiVu®
PPP Loan Data — Diversatek, Inc., Germantown, WI
MiVu® and Diversatek Healthcare AFS Non-CME Presentation September, 2020
Diversatek Healthcare Announces the Commercial Release of MiVu®, First GERD Testing Tool to Detect Esophageal Mucosal Changes Instantly, During Routine Endoscopy
December 1, 2020
MILWAUKEE, Wis. (November 30, 2020) –MiVu®, a first-ever mucosal integrity (MI) testing system from Diversatek Healthcare, was granted De Novo classification* from the Food and Drug Administration (FDA) for the safety and efficacy in obtaining measurements of electrical properties within esophageal tissue. MiVu® is the only system available to clinicians that provides real-time data of mucosal integrity during routine endoscopy, indicating the existence or probability of gastroesophageal reflux disease (GERD) and other esophageal pathologies in just two minutes.
Diversatek Healthcare, in conjunction with Vanderbilt University, developed the MiVu® Mucosal Integrity Testing System to detect changes in esophageal mucosal integrity simply and efficiently. MiVu® utilizes a balloon probe and proprietary software to measure conductivity of the esophageal epithelium directly, while providing clinicians both a color contour map and predictive probability. Its unique design, incorporating both radial and axial impedance sensors, measures mucosal integrity at 180-degree intervals along a 10 cm segment of the esophagus, reducing measurement variability. MiVu® enables clinicians to quickly and efficiently rule out GERD, differentiate primary esophageal disorders like GERD and eosinophilic esophagitis (EoE), and monitor treatment response. It may eliminate the need for ambulatory reflux testing and multiple invasive biopsies.
“There is a lot of symptom overlap between GERD and EoE, said Dr. Michael Vaezi, Professor of Medicine and Otolaryngology at the Vanderbilt University Medical Center in the Division of Gastroenterology, Hepatology and Nutrition. Current diagnostic tests for esophageal disorders, which are often inconclusive, make it challenging to provide patients with reliable, timely information that adequately guides next steps. MiVu® direct measurement provides accurate, instant data that can immediately direct therapy while helping to reduce the need for unnecessary testing.”
“After more than eight years of ongoing testing and clinical studies, we are thrilled to bring MiVu® to the market” said Diversatek Healthcare’s Chairman Brant Stanford. “Current diagnostic tools for patients experiencing chronic esophageal disorders are incapable of detecting subtle, but real, disease conditions. MiVu® is a fast, effective alternative that helps guide therapy decisions and monitors disease activity over time, ultimately saving patients time and money.”
To learn more about MiVu®, please visit https://www.diversatekhealthcare.com/mucosal-integrity-mivu-2/.
Vanderbilt University Medical Center Announces MiVu® - A New Tool to Improve GERD Diagnosis
The MiVu® system is the first of its kind to receive FDA approval. ”We expect direct MI testing to greatly improve the practice of how GERD and other esophageal diseases are diagnosed,” Vaezi said.
Diversatek Healthcare Announces the Commercial Release of MiVu®, First GERD Diagnostic Tool to Detect Esophageal Mucosal Changes Instantly, During Routine Endoscopy
"There is a lot of symptom overlap between GERD and EoE, said Dr. Michael Vaezi, Professor of Medicine and Otolaryngology at the Vanderbilt University Medical Center in the Division of Gastroenterology, Hepatology and Nutrition. Current diagnostic tests for esophageal disorders, which are often inconclusive, make it challenging to provide patients with reliable, timely information that adequately guides next steps. MiVu® direct measurement provides accurate, instant data that can immediately direct therapy while helping to reduce the need for unnecessary testing."